The chemical reactions include the transfer, loss, gain, and sharing of electrons whereas nuclear reactions are characterized by the disintegration of the nucleus and have nothing to do with electrons.
Here are some key distinctions between them to help you learn how they differ:
Chemical Reactions Vs Nuclear Reactions:
What is Chemical Reaction?
In a chemical reaction, there is a loss of neutrons or protons, the nucleus may break down into another atom. Protons and neutrons react within the nucleus.
Chemical reactions, on the other hand, cannot be classified as such.
A material is converted into one or more other substances by the action of electrons in a chemical reaction.
A chemical reaction includes just a little amount of energy change, but a nuclear reaction involves a large amount of energy change.
What is Nuclear Reaction?
In nuclear processes, electrons react just outside of the nucleus in chemical reactions.
Nuclear reactions are classified as fission or fusion. In a nuclear reaction, however, a new element is produced as a result of the proton or neutron’s activity.
In a nuclear reaction, the quantity of energy that varies is 108 kJ. It is 10–103 kJ/mol in chemical reactions.
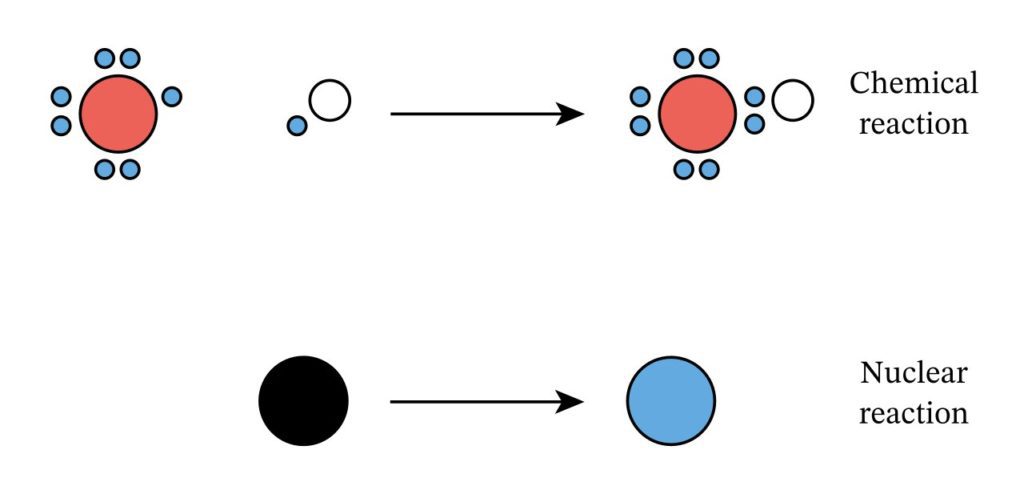
What is the Difference between Chemical Reactions and Nuclear Reactions?
A chemical reaction is a sort of reaction in which two molecules combine or atoms of an element rearrange to generate a completely new product while a nuclear reaction is a sort of reaction in which the structure of an atom’s nucleus changes entirely while releasing energy.
So, what’s the difference? Let’s discuss the difference between chemical and nuclear reactions and how they are similar but not the same.
| Chemical reactions | Nuclear Reactions |
| Chemical reactions often occur outside of the nucleus. | Nuclear reactions take place solely within the nucleus. |
| When chemical reactions occur, elements retain their identity, and the nuclei of atoms stay intact. | The nuclei of atoms change entirely during nuclear processes, and new elements are produced. |
| Pressure and temperature can have an effect on chemical reactions. | A nuclear reaction is unaffected by such circumstances. |
| There is a modest energy shift during such reactions. | Nuclear processes provide considerably greater and bigger energy shifts. |
| Current connections are disrupted and new chemical bonds are established during a chemical reaction. | The nuclear reactions do not include any such activity. |
| Chemical processes can be reversible or irreversible. | Nuclear processes are typically irreversible. |
Conclusion:
As a result of above discussion of the two concepts, you should have a clear understanding of these reactions.
Although nuclear reactions occur in the atom’s nucleus, chemical reactions occur in the atom’s electrons.
When comparing energies, a chemical reaction includes just a little amount of energy change, but a nuclear reaction involves a large amount of energy change.
• Section Under Diff